Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Akino, Norio
Dai-13-Kai Nihon Netsu Bussei Shimpojiumu Koen Rombunshu, p.273 - 276, 1992/00
no abstracts in English
; Nakajima, Hayato; Ikezoe, Yasumasa; ; Shimizu, Saburo
JAERI-M 6139, 35 Pages, 1975/05
no abstracts in English
Nagai, Takayuki; Okamoto, Yoshihiro; Shiwaku, Hideaki; Akiyama, Daisuke*; Sato, Nobuaki*
no journal, ,
PdO reacts to NaNO and rare earth nitrates and Pd compounds is generated in a vitrification process. The reduction behavior of Pd(NO
and rare earth nitrates and Pd compounds is generated in a vitrification process. The reduction behavior of Pd(NO )
) , the synthesis of Pd compounds with Pd(NO
, the synthesis of Pd compounds with Pd(NO )
) and rare earth nitrates at high temperature and chemical reaction of Pd compounds in a molten borosilicate glass were confirmed in this study.
and rare earth nitrates at high temperature and chemical reaction of Pd compounds in a molten borosilicate glass were confirmed in this study.
Kawaguchi, Munemichi
no journal, ,
This study presents the fundamental research on the thermal decomposition behavior of sodium hydride (NaH) in FY2021. This study on the thermal decomposition behavior of NaH was carried out using TG-DTA and FT-IR for the decommissioning of the cold trap in Monju. Due to the weak ionic bonding of Na-H in NaH, the hydrogen is enable to move easily at close to 565K. Upon further heating, the hydrogen desorbs by the thermal decomposition. A simple model using each kinetic rate equations (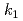 ,
, 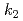 ,
, 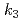 ) can give us the quantitative evaluation of the thermal decomposition behavior of NaH, which tended to overestimated the Na evaporation.
) can give us the quantitative evaluation of the thermal decomposition behavior of NaH, which tended to overestimated the Na evaporation.